Abstract
Background: Atrial fibrillation (AF) is a common disorder that will affect up to 5.6 million patients in the U.S. by 2050. Both direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs), typically warfarin, are used for stroke prevention in AF and such patients frequently undergo invasive procedures requiring anticoagulant interruption. Temporary interruption of anticoagulants can be associated with significant morbidity and mortality in the form of thromboembolic and bleeding complications. DOACs have a short half-life and fast onset of action, thereby facilitating their perioperative management as compared to VKAs. Despite important differences in perioperative management and pharmacokinetics between DOACs and VKAs, there is a paucity of data comparing perioperative outcomes in DOAC and VKA-treated patients.
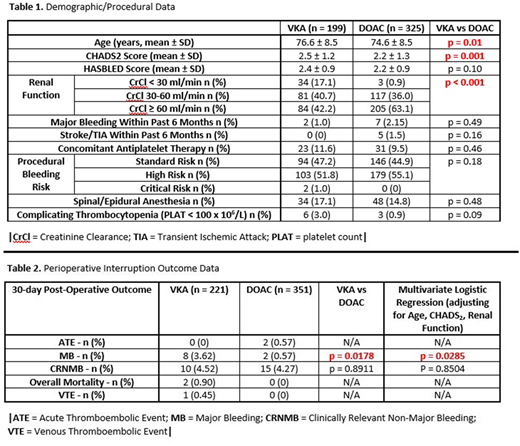
Methods: We undertook a single-center, retrospective chart review that compared consecutive DOAC- or warfarin-treated patients with AF who underwent perioperative anticoagulant interruption for invasive procedures between January 2017 and March 2018. Perioperative warfarin interruption was done as per CHEST guidelines (Douketis et al. Chest 141,2 Suppl). Perioperative bridging with low-molecular-weight heparin was only used for patients with CHADS2 scores of 5-6 or in patients with stroke within the past 6 months. Perioperative interruption of DOACs was done as per Thrombosis Canada guidelines, with anticoagulation held for 3 half-lives prior to low bleeding risk procedures and 5 half-lives for high bleeding risk procedures. Primary outcomes included the 30-day post-operative thromboembolic and major bleeding rates. Secondary outcomes included the 30-day clinically relevant non-major bleeding (CRNMB) andl mortality rates. Major bleeding and CRNMB were defined according to ISTH definitions. Procedural bleeding risk was defined as per Schulman et al (Circulation 2015; 132(3)). Outcome events were independently adjudicated by two investigators. Outcomes from patients on DOACs and VKAs were compared. Demographic data was analyzed on a per-patient basis, p-values were calculated using independent T-Test, Chi-Square/Fisher's Exact Test where appropriate. Outcome data was analyzed on a per-interruption basis. P-values for unadjusted and adjusted comparisons were calculated using generalized estimating equations (GEE) to account for correlation between multiple procedures on the same patients.
Results: 325 DOAC patients and 199 warfarin patients underwent 351 and 221 periprocedural interruptions, respectively. Warfarin patients had a significantly higher mean age, CHADS2 score, and proportion with renal dysfunction (Table 1). There was no statistically significant difference in 30-day post-operative rates of thromboembolism, CRNMB, and overall mortality, but warfarin patients had a significantly higher rate of major bleeding (Table 2). This latter result remained statistically significant following multivariate logistic regression correction for age, CHADS2 score and level of renal dysfunction. All bleeding events occurred post-procedure, with major bleeding events occurring from post-operative day 1 to post-operative day 25. None of the warfarin patients with major bleeding received perioperative bridging; the mean international normalized ratio (INR) at the time of major bleeding was 3.3. Most major bleeding events (7/8) in the VKA arm were surgical, with a single non-surgical major-bleed (spontaneous ICH on post-operative day 15 following urological surgery).
Conclusions: The perioperative interruption of warfarin was associated with a higher 30-day rate of major bleeding as compared with DOAC interruption. Re-initiation of warfarin should be done judiciously following high bleeding risk procedures, and close INR monitoring may be warranted.
Shaw:Portola Pharmaceuticals: Research Funding. Douketis:Janssen: Consultancy; Pfizer: Other: Advisory Board; Boehringer-Ingelheim: Consultancy, Other: Advisory Board, Research Funding; Portola: Other: Advisory Board; The Medicines Company: Other: Advisory Board; Daiichi-Sankyo: Other: Advisory Board; Biotie: Other: Advisory Board; Bayer: Other: Advisory Board; Sanofi: Consultancy, Other: Advisory Board; BMS: Other: Advisory Board; Astra-Zeneca: Other: Advisory Board. Carrier:Bayer: Honoraria; Pfizer: Honoraria; BMS: Honoraria, Research Funding; Leo Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal